- Sommet Education launches its Foundation to support Hospitality sector employment challenges
- Chetu Receives Silver Stevie Award In 2024 American Business Award
- Courtyard by Marriott Aravali Resort x Marriott Bonvoy Unveils 'Resort Escapes'
- Introducing Ettila: The Next Generation Media Platform Redefining Content Discovery
- Lightstorm Marks a Turning Point for NaaS in India at its Flagship event - Blueprint
- Sage Salon Celebrates 15th Anniversary
- BML Munjal University Hosts Sustainable Technology Advancement Conclave 2024
- Revolutionary LazyMonkey Feedback System Launches at Pushpanjali Seasons in Agra
- Acquisty Launches Innovative Digital Marketing Services to Boost Businesses Online
- India's First Multi-Modal AI Aggregator is Soon to be Launched
- CFlo Showcases Sustainable Mineral Beneficiation Solutions at MiningWorld Russia 2024
- Nexval President Talks About Importance Of AI
- Aroma Treasures Announces Wide Range of Natural and Pure Aromatherapy Products
- Wondrlab creates a content campaign for Navratna Oil with Kapil Sharma and team
- Antenna Experts Official Launches Radio Antenna in Canada, USA
 Mail to a Friend Mail to a Friend |
|
     |
Siora's Products are now Registered with the Malaysian Medical Device Authority (MDA)
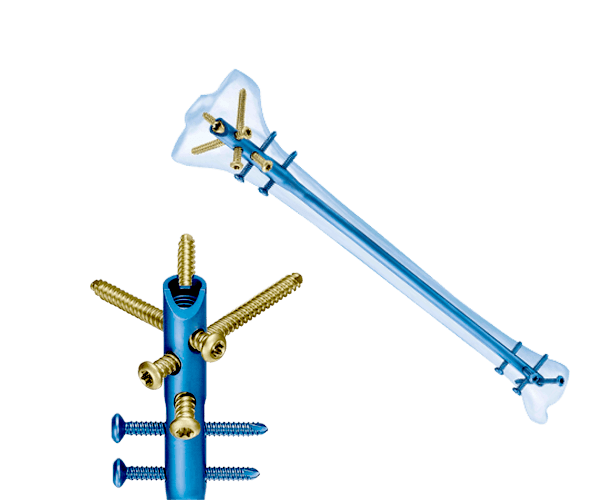
Company's step towards delivering "Value for Money" to clients based in Malaysia.
The demand for high-quality orthopedic implants is increasing in many European and Asian countries. The reason behind this could be a rise in the geriatric population. Malaysia is one of those countries where the requirement for orthopedic implants and instruments is increasing. Plus, the country also relies heavily on imports. The import market of medical devices in Malaysia was around 7,250 million US dollars in2018. This shows how important imports are for Malaysia when it comes to medical devices.
Siora Surgicals Pvt. Ltd., a renowned and experienced manufacturer of a wide range of trauma implants is spreading its wings to establish a good presence in other countries. With more than 30 years of experience in this industry, the company has already created a base of happy & satisfied clients across the globe. Now, to capture a good market share in Malaysia, the implants, instruments, & fixators manufactured by Siora Surgicals are registered with the Malaysian Medical Device Authority (MDA). Receiving this approval means that the orthopedic products of Siora meet all the quality guidelines stated by the concerned authorities in Malaysia. This will also help strengthen the trust with suppliers of orthopedic implants in Malaysia.
The Medical Device Authority is managed by the Ministry of Health Malaysia. The role of this federal statutory agency is to enforce and implement the Medical Device Act 2012 (Act 737). This act mainly focuses on the public health and safety issues concerning medical devices. This ensures trust and reliability of medical devices being used in the country and facilitates the medical device industry and trade. In simpler terms, it could be said that MDA is responsible for enforcing regulations of medical devices being used in Malaysia and ensuring proper medical device registration.
After being registered with the Malaysian Medical Device Authority, Siora Surgicals can now export its International standard quality products to the clients present in the country without any obstacles. The company already has an FDA India-approved manufacturing unit in Sonipat, Haryana (India). Plus, the quality management system (QMS) of the company is set in accordance with WHO-GMP, ISO 9001:2015, and ISO 13485:2016 standards. Siora entered the Malaysian market around 15 years ago, and since then, the company is serving clients with its high-quality products.
Contact Information:
Siora Surgicals Pvt. Ltd
Address ? WZ- 1, 2nd Floor, Phool Bagh,
Ram Pura, New Delhi, 110035 INDIA
+91 9810021264
MANUFACTURING UNIT
Address ? 1792, HSIIDC Industrial Estate,
RAI, District ? Sonipat, Haryana ? 131029
Contact
Anuj Dureja
e-marketing@siora.net
09810021264
Company :-Siora Surgicals Pvt. Ltd
User :- Siora Surgicals
Email :-e-marketing@siora.net
Mobile:- 9810021264
Url :- https://www.siiora.org/









